Last updated: November 6, 2025 · Reading time: 10–14 minutes · Audience: water engineers, RO/boiler/cooling operators
Understanding hardness vs alkalinity lets you infer salt composition, scaling risk, and treatment choices from just two lab numbers. This guide explains the three classification rules, unit conversions, a decision tree from data to chemistry, worked examples, and a practical treatment shortlist.
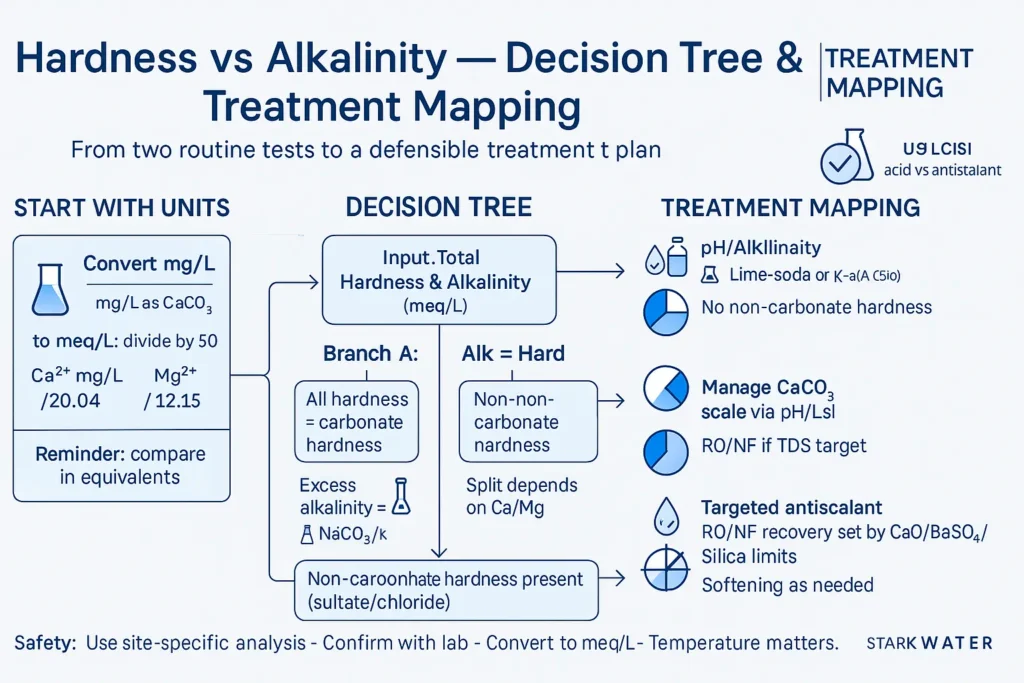
TL;DR — Three Rules to Classify Your Water
Always compare in equivalents (meq/L or mmol/L), not just mg/L as CaCO3. Then apply:
- Alkalinity > Hardness (as equivalents) → all hardness is carbonate hardness; excess alkalinity is sodium/potassium bicarbonate (“negative hardness”).
- Alkalinity = Hardness → Ca/Mg entirely as bicarbonates; no non-carbonate hardness.
- Alkalinity < Hardness → non-carbonate hardness exists (sulfates/chlorides). Split depends on Ca vs Mg share.
Concepts & Units You Actually Need
- Total hardness = Ca2+ + Mg2+ (usually reported as mg/L CaCO3).
- القلوية = acid-neutralizing capacity (HCO3−, CO32−, OH−).
- Carbonate hardness pairs Ca/Mg with bicarbonate/carbonate; non-carbonate hardness pairs them with SO42−/Cl−.
Conversions you will use (copy & keep)
To convert mg/L as CaCO₃ → meq/L: divide by 50
Example: 150 mg/L as CaCO₃ = 150 / 50 = 3.0 meq/L
To convert Ca²⁺ (mg/L) → meq/L: divide by 20.04
To convert Mg²⁺ (mg/L) → meq/L: divide by 12.15
Why equivalents? The rules compare charge balance; mg/L as CaCO₃ is convenient,
but you must convert to meq/L (or mmol/L) to interpret composition correctly.Background references: USGS — Water Hardness - WHO — Water quality resources.
Decision Tree — From Numbers to Salt Composition
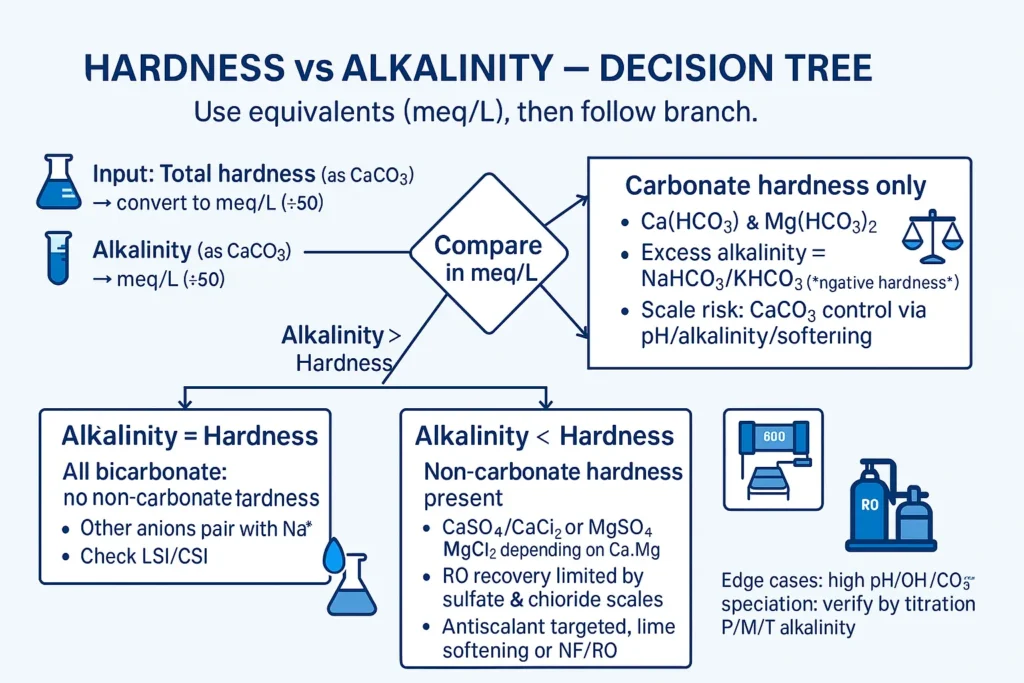
Branch A — Alkalinity > Hardness
All Ca/Mg are present as Ca(HCO3)2/Mg(HCO3)2. Excess HCO3− pairs with Na+/K+ → NaHCO3/KHCO3. There is no non-carbonate hardness; CaSO4 and CaCl2 are absent.
Branch B — Alkalinity = Hardness
Ca/Mg are fully balanced by bicarbonate. Other anions (SO42−, Cl−) pair with Na+/K+ only.
Branch C — Alkalinity < Hardness
Some Ca/Mg must be as sulfates/chlorides. Two useful limiting patterns:
- Ca-dominant hardness: CaSO4 appears; Mg often remains partly as bicarbonate or sulfate.
- Mg-dominant hardness: mix of Mg(HCO3)2 and MgSO4; Ca non-carbonate share can be small.
What It Means in Practice
- Scaling risk: carbonate-only waters favor CaCO3 scale (check LSI/CSI); non-carbonate adds CaSO4/MgSO4 constraints for RO recovery and boiler/cooling cycles.
- Acid vs antiscalant: carbonate-limited systems respond well to pH/alkalinity control; mixed salts often need targeted antiscalant programs.
- Downstream stability: alkalinity affects corrosion indices and post-treatment (e.g., RO permeate remineralization).
General guidance: U.S. EPA water research.
Worked Examples (Swap in Your Data)
| Case | Total hardness | القلوية | Equivalents (meq/L) | Classification | الملاحظات |
|---|---|---|---|---|---|
| A | 150 mg/L | 200 mg/L | Hard = 3.0; Alk = 4.0 | Alk > Hard | All carbonate hardness; “negative hardness” ≈ 50 mg/L as CaCO3 as NaHCO3. |
| B | 180 mg/L | 180 mg/L | Both = 3.6 | Alk = Hard | No non-carbonate hardness; manage CaCO3 scale via pH/Langelier. |
| C | 220 mg/L | 120 mg/L | Hard = 4.4; Alk = 2.4 | Alk < Hard | Non-carbonate hardness = 100 mg/L as CaCO3; expect sulfate/chloride pairs. |
نصيحة: Always compute in meq/L (divide mg/L as CaCO3 by 50) before comparing hardness vs alkalinity.
Testing & Data Quality Checklist
- Run alkalinity titrations promptly; minimize CO2 loss and keep temperature similar across samples.
- Record method (P-/M-/T-alkalinity), endpoints, and detection limits; duplicate critical samples.
- Convert to meq/L and archive spreadsheets for auditability.
Treatment Pathways by Quadrant
Alk > Hard
- Softening (lime-soda) or weak-acid cation for high bicarbonate hardness.
- pH/alkalinity control to manage CaCO3 scaling; consider CO2 balance for stability.
Alk = Hard
- Focus on LSI/CSI; antiscalant optional depending on recovery.
- RO/NF if TDS targets or reuse requirements apply.
Alk < Hard
- Expect CaSO4/MgSO4 limits; select antiscalant by limiting salt.
- RO/NF recovery typically lower; watch boiler/cooling blowdown.
Explore solutions and tools on our site: Water Treatment Solutions - أدوات مياه ستارك ووتر - Case Studies.
Quick Calculator — Carbonate vs Non-Carbonate Split
Use our downloadable sheet to convert mg/L as CaCO3 → meq/L and compute carbonate/non-carbonate hardness automatically.
- Open the online tool
- Download the XLSX (placeholder path)
Get a One-Page Interpretation
Upload your hardness, alkalinity, Ca/Mg split, and anions (Cl−/SO42−). We’ll return a composition breakdown, scaling indices, and a shortlist of treatment routes.
About the Author
Stark Water — Process engineers specializing in scaling control, softening chemistry, RO/NF design, and water quality analytics.
FAQs — Hardness vs Alkalinity
1) Why must I compare in meq/L instead of mg/L as CaCO3 only?
Because the relationship is about charge balance; compare equivalents to interpret composition correctly.
2) What is “negative hardness” and when does it appear?
When alkalinity exceeds hardness; the excess alkalinity is sodium/potassium bicarbonate not paired with Ca/Mg.
3) How do Ca-dominant vs Mg-dominant waters change treatment?
Ca-dominant water often pushes CaCO3/CaSO4 limits; Mg-dominant tends to increase MgSO4 concerns and may respond differently to softening.
4) How does this tie into LSI/CSI and scale control?
Alkalinity and Ca hardness feed directly into LSI/CSI; lowering pH/alkalinity or using antiscalant reduces scaling tendency.
5) Can very high pH (mostly OH−) break the rule?
At very high pH, alkalinity can be dominated by OH−/CO32−. Convert to equivalents and verify speciation—edge cases require full ionic balance.
6) What recovery limits should I check in RO?
Check CaSO4, BaSO4, SrSO4, and silica indices in addition to CaCO3; set recovery to keep them below saturation with safety margins.

