
Disponibilidad: Hay existencias
Ensure the highest standard of water purity for critical healthcare and laboratory needs with Stark Water’s STARK Medical Grade RO System. This advanced medical grade RO system is meticulously engineered to produce ultra-pure water, free from endotoxins and pyrogens, ensuring patient safety and compliance with stringent medical and pharmaceutical regulations.
In medical facilities, pharmaceutical manufacturing, and sensitive research laboratories, water quality is not merely a preference—it is a critical determinant of patient safety, diagnostic accuracy, and product efficacy. Standard purified water is often insufficient. The Stark Water STARK Medical Grade RO System is meticulously engineered to meet and exceed the most stringent purity standards, providing an unwavering supply of ultra-pure water essential for life-saving applications and precision processes.
Our medical grade RO system is designed with a profound understanding of the unique purity requirements of the healthcare and life sciences sectors. Unlike conventional industrial or commercial RO systems, a medical grade RO system is specifically configured to remove not only dissolved solids, heavy metals, and chemical contaminants but also critical microbiological impurities such as bacteria, viruses, pyrogens, and endotoxins. This level of purification is absolutely vital for applications where water directly contacts patients or is used in the preparation of sterile solutions. The system typically incorporates advanced features like:
Every component and design choice is geared towards delivering water that adheres to pharmacopoeia standards (e.g., USP, EP, JP) for Purified Water (PW) or Water for Injection (WFI) pre-treatment, as well as specific medical device processing guidelines (e.g., AAMI for dialysis water).
The construction and operation of the STARK Medical Grade RO System prioritize hygiene and validation capabilities. Key features include:
This meticulous attention to design, material, and process ensures that the medical grade RO system is not only effective but also fully auditable and compliant with the stringent requirements of medical, pharmaceutical, and laboratory environments.
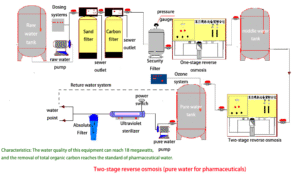
The STARK Medical Grade RO System delivers unwavering reliability, a non-negotiable trait when patient safety or critical research is at stake. Its automated controls, robust components, and comprehensive design minimize the risk of contamination and system failure, providing continuous access to the highest purity water. This ensures optimal performance for dialysis machines, central sterile supply departments, laboratory analyses, and pharmaceutical manufacturing processes, contributing directly to positive patient outcomes and accurate scientific results.
Stark Water’s STARK Medical Grade RO System is engineered for the highest standards of water purity and reliability in critical medical applications. Here are its key technical specifications:
| Parámetro | Especificaciones / Opciones de personalización |
|---|---|
| Palabras clave principales | medical grade RO system design and capabilities. |
| Water Quality Output |
|
| System Configuration | Single or Double Pass Reverse Osmosis (RO) with integrated pre-treatment. Optional post-RO polishing (EDI, UV, Ultrafilters, Deionization). |
| Capacity | Customizable, ranging from 50 LPH to 5000+ LPH (or GPM equivalent), based on application demand (e.g., dialysis clinic, pharmaceutical plant). |
| RO Membrane Type | High-rejection Thin-Film Composite (TFC) RO Membranes, often low-fouling/sanitary-grade. |
| Construction Material (Wetted Parts) | High-grade Stainless Steel (SUS316L, mirror-polished internal surfaces Ra < 0.6 μm or < 0.4 μm). PVDF or PTFE for specific components. |
| Piping | Sanitary Stainless Steel (SUS316L, orbital welded where possible) or PVDF. |
| Disinfection Method | Compatible with Hot Water Sanitization (80-85°C) or Chemical Disinfection (SIP/CIP ready). |
| Sistema de control | Fully Automatic PLC Control with HMI. Comprehensive monitoring of conductivity/resistivity at multiple points, flow, pressure, temperature, TOC, UV intensity. Alarms, data logging, and remote monitoring. |
| Pre-treatment System |
|
| Fuente de alimentación | Trifásico, 380V/415V/460V, 50Hz/60Hz (personalizable). |
| Certifications & Standards | Designed and manufactured to meet industry standards like USP (United States Pharmacopeia), EP (European Pharmacopoeia), JP (Japanese Pharmacopoeia) for PW/WFI, AAMI (Association for the Advancement of Medical Instrumentation) for dialysis water, ISO, CE, FDA/GMP compliance. |
| Dimensions | Customized skid-mounted or modular design to fit cleanroom or facility requirements. |
En STARK Medical Grade RO System is widely adopted across various sectors where clean, microbiologically safe, and low-conductivity water is critical. Our Two-stage RO platform is flexible enough to support applications ranging from small clinic equipment to centralized hospital sterilization.
In each of these settings, STARK’s systems are customized to meet space limitations, operational load, and water safety regulations — offering both performance and peace of mind.
In critical medical environments, water quality isn't optional—it’s essential. STARK’s dual-stage reverse osmosis architecture offers a robust solution to meet the highest purity standards for clinical and pharmaceutical use.
The first-stage RO system removes most dissolved solids, microorganisms, and organic substances. The second-stage RO further polishes the water by eliminating residual ions, pyrogens, and any potential microbial breakthrough from the first pass. This ensures stable production of ultrapure water with:
With this Two-stage process, our systems offer consistent water quality for critical departments such as dialysis, CSSD, laboratories, and compounding pharmacies. The separation efficiency and safety margin help ensure full compliance with international medical water requirements.
La vida útil de un sistema de ósmosis inversa depende de la calidad del agua y del mantenimiento. Normalmente, la membrana dura entre 2 y 3 años, mientras que el sistema en sí puede durar más de 10 años con los cuidados adecuados.
Sí, todos nuestros sistemas son totalmente personalizables. Analizamos su informe de aguas y sus necesidades de aplicación antes de ofrecerle soluciones a medida.
Nuestros productos cumplen las normas CE e ISO 9001, y pueden diseñarse para cumplir normativas regionales o estándares industriales específicos si así se solicita.
Los sistemas estándar se envían en un plazo de 7-15 días. Para los pedidos personalizados, el plazo de entrega puede prolongarse hasta 20-25 días en función de la complejidad.
Sí. Proporcionamos documentación técnica completa, asistencia remota por vídeo y podemos enviar ingenieros al extranjero si así se solicita.
Nuestro presupuesto incluye el sistema de ósmosis inversa completo, el panel de control, las bombas, las unidades de pretratamiento y todos los accesorios necesarios. Si lo desea, podemos incluir herramientas de instalación y piezas de repuesto.
Persona de contacto:Zora Jiang
Teléfono:+86-18520151000
Correo electrónico:stark@stark-water.com
Dirección:Edificio C, Longchuang Micro-Chuangyuan, #26 Hantang Street, Distrito de Dongcheng, Ciudad de Dongguan. China.

Disponibilidad: Hay existencias