This guide explains what an aseptic stainless steel water tank is, how to design and size it for pharmaceutical, beverage and pure-water duties, and—most importantly—how to implement CIP 그리고 SIP correctly. We also provide checklists, nozzle matrices, and links to related RO systems and membranes so you can move from concept to RFQ fast.

Need a ready-to-quote spec? Browse aseptic tank models or pair with our 1000 LPH RO System.
1) What Is an Aseptic / Sterile Water Tank?
An aseptic stainless steel water tank stores purified process water with minimal bioburden risk. Compared with generic “hygienic” tanks, aseptic tanks use 304/316L materials, controlled finishes (often ≤0.4 µm RA), crevice-free geometry, validated spray devices, and provisions for CIP (clean-in-place) and SIP (steam-in-place).
Typical water types include PW/HPW from RO or RO+EDI systems. For WFI or other ultra-critical duties, validation depth is confirmed per project.For regulated plants, an aseptic stainless steel water tank reduces bioburden risk between RO/EDI and points of use.
2) Where It’s Used
- Pharma & biotech — PW buffer/storage & distribution
- Beverage & dairy — blend/ingredient water, CIP make-up
- Food & cosmetics — sanitary storage before processing
- Industrial pure water — RO/EDI permeate buffer
Pair with upstream RO packages such as our 1000 LPH Reverse Osmosis System and membrane selections from RO membranes (4040/8040) — e.g. 4040 BWRO ULP31-4040.Choosing an aseptic stainless steel water tank over generic sanitary vessels simplifies validation and routine CIP.
3) Core Design Principles
The geometry and surface finish of an aseptic stainless steel water tank determine cleanability and long-term performance.

3.1 Materials & Fabrication
- 304 vs 316L: Choose 316L for chloride-rich environments; 304 suits many PW cases.
- Pickling & passivation: Restore passive layer post-fabrication.
- Electropolishing (EP) optional: Improves RA and cleanability.
- Weld quality: Smooth ground welds; keep WPS/PQR and a weld map in QA.
3.2 Surface Finish & Cleanability
| Finish Level | Typical RA (µm) | Method | Typical Duty |
|---|---|---|---|
| Standard sanitary | ≤0.8 | 2B/BA + mechanical polish | Food & beverage, general PW |
| High sanitary | ≤0.4 | Fine polish / electropolish | Pharma PW/HPW, high hygiene |
3.3 Geometry & Drainability
- Sloped-to-drain bottom; avoid ledges/pockets; crevice-free internals.
- Minimize dead-legs on nozzles/instrument tees by good-practice ranges.
- Top-entry sanitary manway; sampling valve placed for representative draws.
3.4 Thermal & Mechanical
- Jacket/insulation options; validate for SIP if applicable.
- Operating temp/pressure limits; supports for lifting, transport, seismic.
4) Fittings & Instrumentation (What to Specify)
- Sanitary manway; 0.2 µm hydrophobic vent filter with P/V valve and integrity test port.
- Spray device (static vs rotary) sized for CIP coverage; see CIP section.
- Level (magnetic/radar), temperature (RTD), pressure, sampling valve, sight glass.
- Sanitary connections per DIN/SMS/ISO; gaskets compatible with cleaning chemicals.
A correctly sized vent filter keeps the aseptic stainless steel water tank under sterile conditions during CIP/SIP cycles.
| Nozzle | DN | Standard | Purpose | Location |
|---|---|---|---|---|
| Vent & sterile filter | DN25–DN40 | DIN | Breathing, SIP venting, integrity test | Top |
| CIP spray device | DN40–DN50 | DIN | CIP liquid inlet | Top |
| Feed/return | DN32–DN50 | DIN | Inlet/outlet to distribution | Side/Bottom |
| Drain | DN40 | DIN | Complete drain | Bottom |
| Sampling | DN15 | DIN | QC sampling | Side |
5) CIP & SIP: Cleaning and Sterilization (Deep Dive)
The heart of an aseptic stainless steel water tank is a robust, validated CIP program—and, when required, SIP. Below is a practical sequence and key setpoints to include in your URS and FAT/SAT.During alkaline and acid phases, recirculate through the aseptic stainless steel water tank until conductivity endpoints are met.
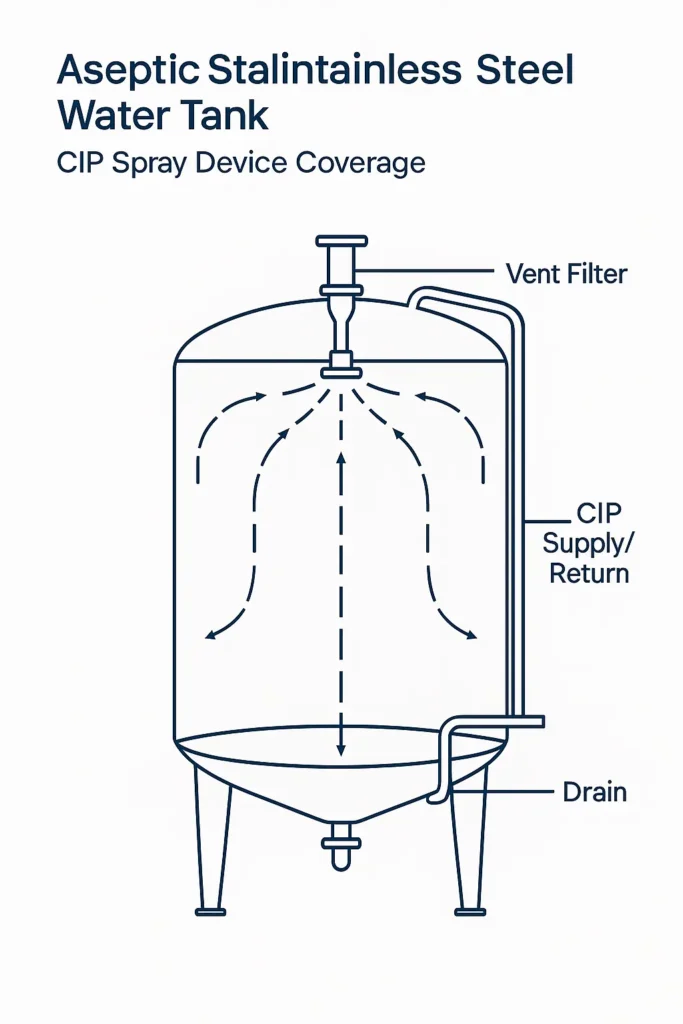
5.1 Spray Device Selection & Coverage
- Static spray ball — fewer moving parts; ensure min. flow/pressure for sheeting.
- Rotary spray — more mechanical action; verify impact pressure and full coverage at operating flow.
- Coverage test — riboflavin (or equivalent) during FAT.
5.2 CIP Hardware & Setpoints (Typical)
- Flow/pressure: Size CIP pump for spray device min. specs at tank head; include line losses.
- 온도: 40–70 °C for alkaline/acid phases (project-specific).
- Chemistry: Alkaline → Rinse → Acid → Final rinse → Optional passivation.
- Timing: Pre-rinse 5–10 min → Alkaline 10–20 min → Rinse 5–10 min → Acid 10–20 min → Final rinse 5–10 min.
- Endpoints: Inline conductivity for rinses; add TOC monitoring if URS requires.
5.3 SIP (Steam-In-Place) Basics
- Setpoint: ~121 °C hold 20–30 min measured at the cold spot.
- Venting/condensate: Use sterile vent filter line; drain condensate; avoid slugging.
- Cooling: Controlled cool-down; correct P/V valve setting to prevent collapse.
- Filter integrity: Post-SIP integrity test (e.g., bubble point) per supplier.
5.4 Validation & Documentation
- FAT: Spray coverage, CIP flow/pressure/temp profiles, instrument calibration.
- SAT/IQ/OQ: Replicate cycles onsite; verify acceptance criteria.
- SOPs: Recipes, chemical safety, weekly/monthly checks.
Related resources: 1000 LPH RO System - Industrial Water Treatment Packages - RO Membranes 4040/8040
6) Sizing & Selection Workflow
Step 1 — Volume: Required volume = (peak demand L/h × buffer time h) × safety factor (1.2–1.5). Keep working volume below total volume to allow headspace.
Step 2 — Material & Finish: 304 vs 316L; RA target; electropolish if required by QA.
Step 3 — Fittings package: Vent filter, spray device, level/temperature, sampling, sight glass; DIN/SMS/ISO standards.
Step 4 — Thermal/mechanical: Jacket/insulation; operating temp/pressure; supports and seismic per site.
When sizing an aseptic stainless steel water tank, consider buffer time, headspace for thermal expansion, and future demand.
| Nominal Volume | Footprint (approx.) | Finish | 참고 |
|---|---|---|---|
| 1,000 L | ~Ø1.1 m × 1.6 m | RA ≤0.8 µm (opt: ≤0.4) | Skid or standalone |
| 10,000 L | ~Ø2.2 m × 3.2 m | RA ≤0.8 / ≤0.4 | Field handling plan needed |
| 30,000 L | ~Ø3.1 m × 4.8 m | RA ≤0.8 / ≤0.4 | Seismic check, transport routing |
| 100,000 L | Project-specific | RA per URS | Modular sections, on-site assembly |
7) QA / Documentation & Testing
Request surface-roughness reports for the aseptic stainless steel water tank along with weld maps and hydrostatic tests.
- Material certificates (EN 10204 3.1), weld map, WPS/PQR.
- Surface-roughness report, hydrostatic test, dye-penetrant if required.
- FAT/SAT protocols and reports; IQ/OQ templates (project level).
8) Compliance & Guidelines (High-Level)
Sanitary design follows good manufacturing/food practices and industry guidelines. Exact codes and validation depth are confirmed per project and jurisdiction.
9) Stainless vs FRP/PE: When Steel Wins
- Superior cleanability and thermal tolerance; wide process acceptance in regulated industries.
- Mechanical integrity and lifespan; fewer concerns at elevated temperature.
- TCO advantages when factoring cleaning cycles, downtime, compliance docs.
10) Cost & Lead Time Drivers
- Finish level (≤0.8 vs ≤0.4 µm), 316L, electropolish.
- Nozzle/instrument package; jacket & insulation; testing scope; documentation set.
- Logistics: size, on-site access, seismic requirements.
11) Case Snapshots
- Pharma PW Buffer 10 m³: 316L, RA≤0.4 µm, static spray ball, SIP 121 °C/30 min; FAT+SAT completed.
- Beverage Blend 30 m³: 304, RA≤0.8 µm, rotary spray; jacketed & insulated; inline conductivity control.
- Pure Water 1 m³: 304, RA≤0.8 µm; paired with 1000 LPH RO; compact footprint.
An aseptic stainless steel water tank with validated CIP/SIP is the safe baseline for modern PW/HPW systems.
3-A Sanitary Standards (overview)
EHEDG hygienic design principles
ASME BPE (Bioprocessing Equipment) overview
12) Downloads & RFQ
Prefer a spec pack (GA + P&ID + Material List)? Contact us to receive a bundle tailored to your flow and finish requirements.
13) FAQs
What RA value do I need for pharmaceutical water?
RA ≤0.4 µm is commonly specified for higher sanitary duties; confirm with QA and validation plan.
304 or 316L for chloride-rich environments?
316L provides better pitting resistance; evaluate chloride level, temperature and CIP chemistry.
Can the tank be SIP sterilized and at what conditions?
Yes. Typical regime is ~121 °C for a 20–30 min hold measured at the cold spot, with proper venting and condensate drain; perform vent filter integrity test after SIP.
How do I size the tank for peak demand vs average flow?
Required volume = (peak demand L/h × buffer hours) × safety factor (1.2–1.5). Keep working volume below total to allow headspace.
What documents should I request before FAT?
Material certs (EN 10204 3.1), weld map, WPS/PQR, surface-roughness report, hydrostatic test, CIP coverage validation, instrument calibration sheets.

