
STARK 의료용 RO 시스템
Ensure the highest standard of water purity for critical healthcare and laboratory needs with Stark Water’s STARK Medical Grade RO System. This advanced medical grade RO system is meticulously engineered to produce ultra-pure water, free from endotoxins and pyrogens, ensuring patient safety and compliance with stringent medical and pharmaceutical regulations.
제품 설명
In medical facilities, pharmaceutical manufacturing, and sensitive research laboratories, water quality is not merely a preference—it is a critical determinant of patient safety, diagnostic accuracy, and product efficacy. Standard purified water is often insufficient. The Stark Water STARK Medical Grade RO System is meticulously engineered to meet and exceed the most stringent purity standards, providing an unwavering supply of ultra-pure water essential for life-saving applications and precision processes.
The Uncompromising Purity of a Medical Grade RO System
Our medical grade RO system is designed with a profound understanding of the unique purity requirements of the healthcare and life sciences sectors. Unlike conventional industrial or commercial RO systems, a medical grade RO system is specifically configured to remove not only dissolved solids, heavy metals, and chemical contaminants but also critical microbiological impurities such as bacteria, viruses, pyrogens, and endotoxins. This level of purification is absolutely vital for applications where water directly contacts patients or is used in the preparation of sterile solutions. The system typically incorporates advanced features like:
- Multi-stage Purification: Often includes double-pass RO or integrated post-treatment technologies like Electrodeionization (EDI) to achieve extremely low conductivity and high resistivity.
- Sterile Filtration & Disinfection: Utilizes ultrafilters or UV sterilizers as final safeguards against microorganisms and endotoxins.
- Recirculation Loops: Designed to prevent water stagnation, minimizing biofilm formation and maintaining high water quality within distribution systems.
Every component and design choice is geared towards delivering water that adheres to pharmacopoeia standards (e.g., USP, EP, JP) for Purified Water (PW) or Water for Injection (WFI) pre-treatment, as well as specific medical device processing guidelines (e.g., AAMI for dialysis water).
Hygienic Design and Validation for Critical Applications
The construction and operation of the STARK Medical Grade RO System prioritize hygiene and validation capabilities. Key features include:
- Sanitary Construction Materials: All wetted parts are typically made from high-grade stainless steel (SUS316L) with mirror-polished internal surfaces (e.g., Ra < 0.6 μm) to prevent microbial adhesion and facilitate thorough cleaning.
- Crevice-Free Welding: Orbital welding ensures smooth, crevice-free joints that eliminate potential harborage sites for bacteria and biofilm.
- Comprehensive Monitoring: Advanced instrumentation for real-time monitoring of critical parameters like conductivity, TOC (Total Organic Carbon), temperature, and flow, ensuring continuous compliance.
- Easy Disinfection & Sterilization: Designed to be fully compatible with chemical or hot water disinfection/sterilization-in-place (SIP) procedures, crucial for maintaining aseptic conditions.
- Documentation & Traceability: Provided with extensive documentation for validation purposes, including material certificates, weld logs, and testing reports, supporting regulatory compliance.
This meticulous attention to design, material, and process ensures that the medical grade RO system is not only effective but also fully auditable and compliant with the stringent requirements of medical, pharmaceutical, and laboratory environments.
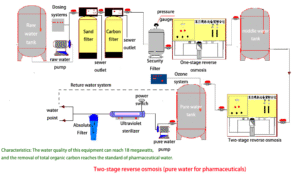
Reliability for Patient Safety and Critical Processes
The STARK Medical Grade RO System delivers unwavering reliability, a non-negotiable trait when patient safety or critical research is at stake. Its automated controls, robust components, and comprehensive design minimize the risk of contamination and system failure, providing continuous access to the highest purity water. This ensures optimal performance for dialysis machines, central sterile supply departments, laboratory analyses, and pharmaceutical manufacturing processes, contributing directly to positive patient outcomes and accurate scientific results.
제품 매개변수
Stark Water’s STARK Medical Grade RO System is engineered for the highest standards of water purity and reliability in critical medical applications. Here are its key technical specifications:
| 매개변수 | 사양 / 사용자 지정 옵션 |
|---|---|
| 핵심 키워드 포커스 | medical grade RO system design and capabilities. |
| Water Quality Output |
|
| System Configuration | Single or Double Pass Reverse Osmosis (RO) with integrated pre-treatment. Optional post-RO polishing (EDI, UV, Ultrafilters, Deionization). |
| 용량 | Customizable, ranging from 50 LPH to 5000+ LPH (or GPM equivalent), based on application demand (e.g., dialysis clinic, pharmaceutical plant). |
| RO 멤브레인 유형 | High-rejection Thin-Film Composite (TFC) RO Membranes, often low-fouling/sanitary-grade. |
| Construction Material (Wetted Parts) | High-grade Stainless Steel (SUS316L, mirror-polished internal surfaces Ra < 0.6 μm or < 0.4 μm). PVDF or PTFE for specific components. |
| Piping | Sanitary Stainless Steel (SUS316L, orbital welded where possible) or PVDF. |
| Disinfection Method | Compatible with Hot Water Sanitization (80-85°C) or Chemical Disinfection (SIP/CIP ready). |
| 제어 시스템 | Fully Automatic PLC Control with HMI. Comprehensive monitoring of conductivity/resistivity at multiple points, flow, pressure, temperature, TOC, UV intensity. Alarms, data logging, and remote monitoring. |
| 전처리 시스템 |
|
| 전원 공급 장치 | 3상, 380V/415V/460V, 50Hz/60Hz(사용자 지정 가능). |
| Certifications & Standards | Designed and manufactured to meet industry standards like USP (United States Pharmacopeia), EP (European Pharmacopoeia), JP (Japanese Pharmacopoeia) for PW/WFI, AAMI (Association for the Advancement of Medical Instrumentation) for dialysis water, ISO, CE, FDA/GMP compliance. |
| 치수 | Customized skid-mounted or modular design to fit cleanroom or facility requirements. |
해당 산업
그리고 STARK 의료용 RO 시스템 is widely adopted across various sectors where clean, microbiologically safe, and low-conductivity water is critical. Our Two-stage RO platform is flexible enough to support applications ranging from small clinic equipment to centralized hospital sterilization.
- Dialysis Centers: Provides high-purity feed water for hemodialysis machines. Two-stage RO ensures the removal of endotoxins, bacteria, and heavy metals to protect patient safety.
- Hospital CSSD (Central Sterile Supply Department): RO water is used for cleaning, rinsing, and steam generation in autoclaves, minimizing the risk of mineral deposits and microbial residue on surgical instruments.
- Clinical Laboratories: Supports reagent preparation, sample dilution, and device rinsing. Low TDS levels improve testing accuracy and extend analyzer lifespan.
- Pharmaceutical Production: Acts as pre-treatment before ultra-pure water systems or is used directly for non-injection cleaning and formulation processes.
- Research Institutes & IVF Centers: Ensures contamination-free water for cell culture, DNA extraction, and sensitive biological reactions.
- Mobile Medical Units / Field Clinics: Offers compact water purification solutions with generator/solar power options, suitable for remote or emergency healthcare deployment.
In each of these settings, STARK’s systems are customized to meet space limitations, operational load, and water safety regulations — offering both performance and peace of mind.
장점 1
In critical medical environments, water quality isn't optional—it’s essential. STARK’s dual-stage reverse osmosis architecture offers a robust solution to meet the highest purity standards for clinical and pharmaceutical use.
The first-stage RO system removes most dissolved solids, microorganisms, and organic substances. The second-stage RO further polishes the water by eliminating residual ions, pyrogens, and any potential microbial breakthrough from the first pass. This ensures stable production of ultrapure water with:
- Conductivity as low as 1–2 μS/cm
- Endotoxin reduction to below 0.25 EU/mL
- Total Dissolved Solids (TDS) removal rate > 98%
- TOC reduction < 50 ppb
With this Two-stage process, our systems offer consistent water quality for critical departments such as dialysis, CSSD, laboratories, and compounding pharmacies. The separation efficiency and safety margin help ensure full compliance with international medical water requirements.
이점 2
관련 제품
연관 상품
자주 묻는 질문
Q1: 역삼투압 시스템의 수명은 얼마나 되나요?
역삼투압 시스템의 수명은 수질과 유지 관리에 따라 달라집니다. 일반적으로 멤브레인의 수명은 2~3년이며, 시스템 자체는 적절한 관리를 통해 10년 이상 지속될 수 있습니다.
Q2: 다양한 수질 조건에 따라 시스템을 맞춤 설정할 수 있나요?
예, 모든 시스템은 완벽하게 사용자 정의할 수 있습니다. 고객의 수질 보고서와 애플리케이션 요구 사항을 분석하여 맞춤형 솔루션을 제공합니다.
Q3: STARK 장비는 어떤 표준을 준수하나요?
당사의 제품은 CE, ISO 9001을 준수하며 요청에 따라 특정 지역 규정 또는 산업 표준을 충족하도록 설계할 수 있습니다.
Q4: STARK RO 시스템의 배송 시간은 어떻게 되나요?
표준 시스템은 7~15일 이내에 배송됩니다. 맞춤형 주문의 경우 복잡성에 따라 리드 타임이 20~25일로 연장될 수 있습니다.
Q5: 기술 지원이나 설치 안내를 제공하나요?
예. 전체 기술 문서와 비디오를 통한 원격 지원을 제공하며, 요청 시 엔지니어를 해외로 파견할 수 있습니다.
Q6: 견적에는 무엇이 포함되나요?
견적에는 전체 RO 시스템, 제어판, 펌프, 전처리 장치 및 필요한 모든 피팅이 포함됩니다. 요청 시 설치 도구와 예비 부품도 포함될 수 있습니다.








