Last updated: November 2025 · Reading time: 12–16 minutes · Audience: plant engineers, EPCs, O&M teams
Why does RO permeate pH differ from feed? This guide explains carbonate buffering, gas/ion selectivity, ammonia speciation, and how degassing, caustic trim, or two-pass RO control permeate pH reliably—without creating scaling or corrosion risks.
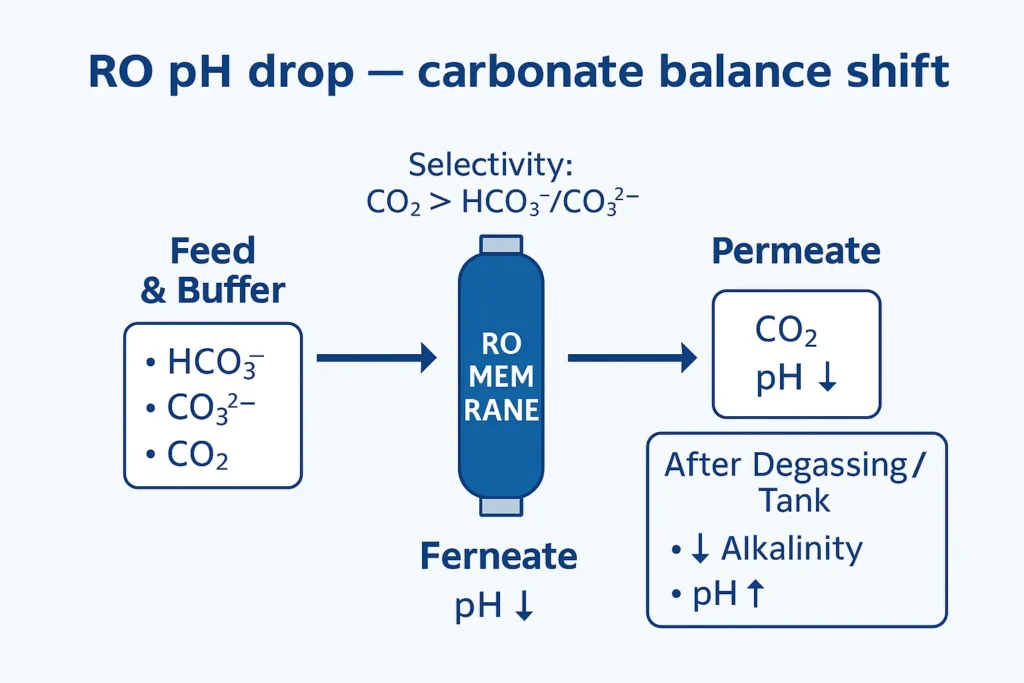
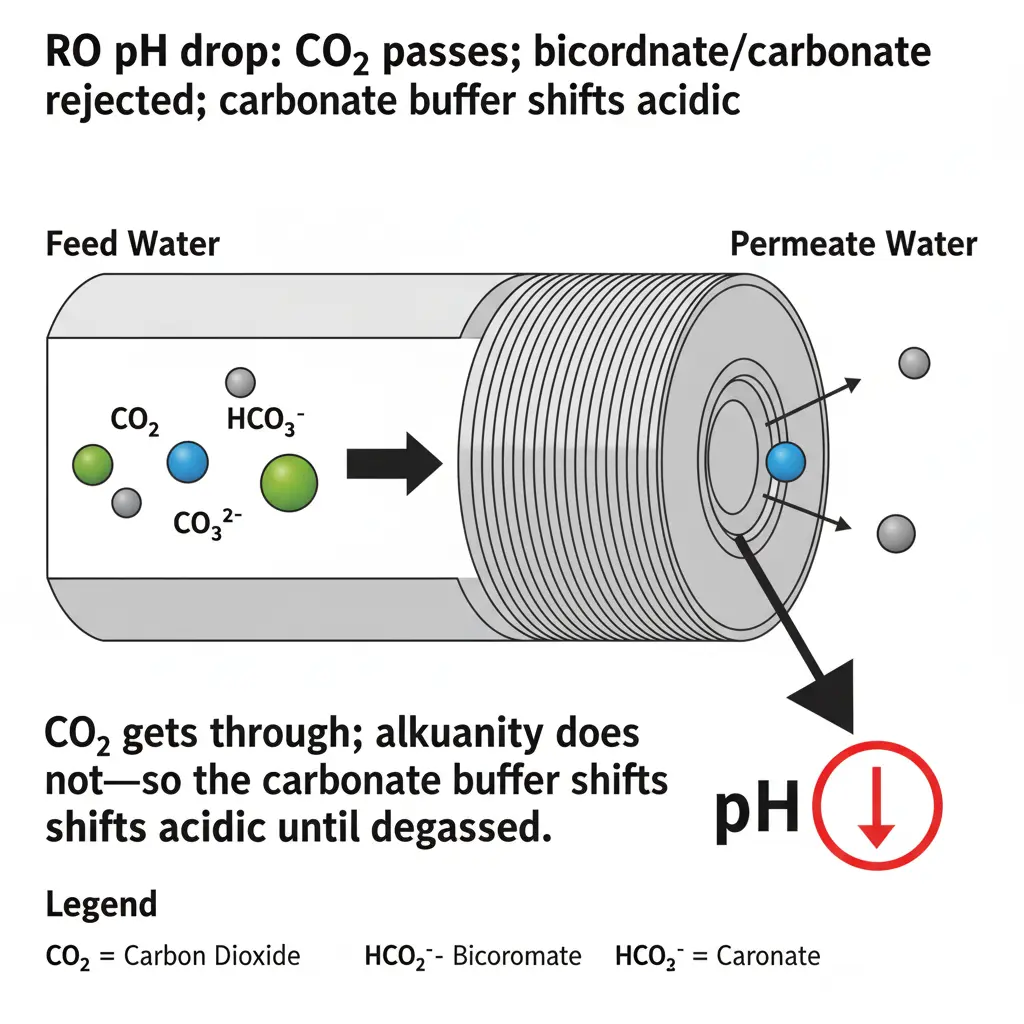
Operators often ask why RO permeate pH falls below neutral even when feed pH looks fine. The short answer is that RO permeate pH reflects gas/ion selectivity: CO₂ slips through while alkalinity stays behind, so the carbonate buffer shifts acidic until CO₂ is vented.
Executive Summary — What Changes and Why
- RO does not “add acid or caustic” by itself—it selectively transports species. Neutral gases (e.g., CO₂, NH₃) permeate more easily than ions (HCO₃⁻/CO₃²⁻, NH₄⁺).
- pH typically drops when CO₂ passes but alkalinity is rejected; the carbonate buffer re-equilibrates to an acidic side.
- pH can rise in ammonia waters because NH₃ passes, NH₃ + H₂O ⇌ NH₄⁺ + OH⁻ → net alkaline permeate.
- Control is straightforward: degassing + caustic trim for CO₂ cases; acidify/strip for ammonia; two-pass RO when boron/low-TDS limits apply.
Where to Measure Permeate pH (and Why Values Differ)
- Inline permeate line: raw effect of separation (often the lowest pH in CO₂-rich waters).
- Permeate tank / after degassing: pH moves upward as CO₂ is stripped and the buffer shifts.
- Post-neutralization: final spec; confirm stabilization and avoid air pickup in sampling.
Best practice: calibrate the pH probe (2- or 3-point), allow stabilization at constant flow/pressure, record temperature and conductivity together.
Carbonate System 101 — The Buffer You Disrupted
In most natural waters, pH between ~6.3 and 9 is set by the CO₂/H₂CO₃/HCO₃⁻/CO₃²⁻ system. RO rejects ions (HCO₃⁻, CO₃²⁻) far more than it rejects dissolved CO₂. Permeate leaves the module with low alkalinity but residual CO₂—so the equilibrium shifts acidic until CO₂ is vented.
| Scenario | Mechanism | Typical symptoms | Primary fixes |
|---|---|---|---|
| A. pH lower than feed (most common) | CO₂ permeates; alkalinity rejected → buffer collapses acidic until CO₂ strips | Inline permeate pH ≈ 5.2–6.5; rises after vent/degassing tank | Forced-draft/packed-tower/vacuum degassing → NaOH trim to spec; consider two-pass RO when boron limits apply |
| B. pH higher than feed (ammonia waters) | NH₃ (neutral) permeates; NH₄⁺ rejected. NH₃ hydrolysis produces OH⁻ | Inline permeate pH ≥ feed; conductivity stays low; may smell of ammonia | Mild acidification to shift NH₃→NH₄⁺ upstream; air/steam stripping or biological route; controlled CO₂ re-dose only if required downstream |
Diagnostics — A Quick Operator Playbook
- Sampling plan: feed, concentrate, inline permeate, tank, post-neutralization; add per-stage permeate if troubleshooting.
- Log with context: pH, temperature, alkalinity, conductivity/TDS, total/free CO₂ (calc), NH₃/NH₄⁺ (or TAN), tank vent status, degasser airflow.
- Normalize: trend salt rejection and ΔP by temperature; for pH, compare same point day-to-day.
- Sanity-check chemistry:
- CO₂ tendency: high alkalinity, high recovery, warm water, closed tank.
- NH₃ tendency: TAN present, higher feed pH, chloramination history.
RO Permeate pH Control Strategies — Map Cause to Remedy
When CO₂ drives low permeate pH
- Degassing: packed column, forced-draft or vacuum units; ensure venting on permeate tank headspace.
- Caustic neutralization: VFD-controlled NaOH/Na₂CO₃ dosing with interlocks; track LSI/SDI to avoid overshoot and post-RO scaling.
- Two-pass RO (RO-II): degas + caustic between passes; improves boron removal and stabilizes pH for high-purity specs.
When NH₃ drives high permeate pH
- Upstream acidification: push speciation to NH₄⁺ (ion, rejected by RO). Avoid over-acidifying to where corrosion rises or antiscalant loses efficacy.
- Strip or biologically remove ammonia: air/steam stripping or biological routes as applicable.
- Trim back down only when needed: gentle CO₂ re-dose or blend; check disinfectant strategy to avoid DBP spikes.
Always confirm material compatibility (polyamide chlorine limits, pH/temperature bounds) and keep compliance with local discharge or product-water regulations.
Worked Examples
Example 1 — Surface Water with High Alkalinity
Inline permeate pH ≈ 5.8 due to CO₂ carry-through; after packed-tower degassing, pH rises to 6.8–7.1. A small NaOH trim brings distribution to pH 7.2–7.5 without positive LSI.
Example 2 — Ammonia-Bearing Groundwater
Feed TAN = 2–5 mg/L as N; inline permeate pH > feed because NH₃ permeates and hydrolyzes. Mild upstream acidification (target pH ≈ 6.5–6.8) shifts TAN to NH₄⁺; permeate pH normalizes around neutral without excess chloride dose.
Figures (add after upload)
Need Help Stabilizing RO Permeate pH?
Send feed analysis (alkalinity, pH, CO₂, TAN (NH₃/NH₄⁺), температура, SDI) and your product-water target. Our engineers will simulate pH drift, size a degasser or neutralization skid, and deliver a defensible process train.
If your RO permeate pH is consistently out of spec, trend where it is measured (inline, tank, post-neutralization) and log alkalinity, CO₂ and TAN. Stabilizing RO permeate pH usually takes a simple combination of degassing and trim dosing with proper interlocks.
Water Treatment Solutions - Case Studies - Водные инструменты Stark - Блог - Запрос Цитировать
References & Further Reading
- U.S. EPA — Water Research: Drinking Water & Treatment
- WHO — Guidelines for Drinking-water Quality (GDWQ)
- USGS — Alkalinity and Water (carbonates & buffering)
About the Author
Stark Water — process engineers focused on UF/NF/RO diagnostics, degassing & neutralization design, and lifecycle-cost optimization.
FAQs — RO Permeate pH
1) Why isn’t RO permeate pH equal to feed pH?
Because RO transports gases and ions differently. CO₂ and NH₃ can permeate; alkalinity and NH₄⁺ are largely rejected, shifting the buffer balance.
2) Will raising permeate pH increase scaling risk?
Yes, if you overshoot. Track LSI/SI with temperature and add trim dosing under interlocks. Degassing + small NaOH is safer than heavy caustic alone.
3) Should I neutralize before or after the permeate tank?
After degassing/venting. Let CO₂ escape first, then trim to spec; otherwise you dose more than needed and risk post-tank pH rebound.
4) Can RO remove CO₂ or NH₃ by itself?
No. RO rejects ions effectively but neutral gases slip through. Use degassing for CO₂ and acidification/stripping for ammonia control.
5) When is two-pass RO justified?
When tight boron or low-TDS limits apply, or when you need a very stable product pH with minimal chemicals—often paired with inter-pass degassing and caustic.

