
STARK Medical Grade RO System
Ensure the highest standard of water purity for critical healthcare and laboratory needs with Stark Water’s STARK Medical Grade RO System. This advanced medical grade RO system is meticulously engineered to produce ultra-pure water, free from endotoxins and pyrogens, ensuring patient safety and compliance with stringent medical and pharmaceutical regulations.
Beschreibung des Produkts
In medical facilities, pharmaceutical manufacturing, and sensitive research laboratories, water quality is not merely a preference—it is a critical determinant of patient safety, diagnostic accuracy, and product efficacy. Standard purified water is often insufficient. The Stark Water STARK Medical Grade RO System is meticulously engineered to meet and exceed the most stringent purity standards, providing an unwavering supply of ultra-pure water essential for life-saving applications and precision processes.
The Uncompromising Purity of a Medical Grade RO System
Our medical grade RO system is designed with a profound understanding of the unique purity requirements of the healthcare and life sciences sectors. Unlike conventional industrial or commercial RO systems, a medical grade RO system is specifically configured to remove not only dissolved solids, heavy metals, and chemical contaminants but also critical microbiological impurities such as bacteria, viruses, pyrogens, and endotoxins. This level of purification is absolutely vital for applications where water directly contacts patients or is used in the preparation of sterile solutions. The system typically incorporates advanced features like:
- Multi-stage Purification: Often includes double-pass RO or integrated post-treatment technologies like Electrodeionization (EDI) to achieve extremely low conductivity and high resistivity.
- Sterile Filtration & Disinfection: Utilizes ultrafilters or UV sterilizers as final safeguards against microorganisms and endotoxins.
- Recirculation Loops: Designed to prevent water stagnation, minimizing biofilm formation and maintaining high water quality within distribution systems.
Every component and design choice is geared towards delivering water that adheres to pharmacopoeia standards (e.g., USP, EP, JP) for Purified Water (PW) or Water for Injection (WFI) pre-treatment, as well as specific medical device processing guidelines (e.g., AAMI for dialysis water).
Hygienic Design and Validation for Critical Applications
The construction and operation of the STARK Medical Grade RO System prioritize hygiene and validation capabilities. Key features include:
- Sanitary Construction Materials: All wetted parts are typically made from high-grade stainless steel (SUS316L) with mirror-polished internal surfaces (e.g., Ra < 0.6 μm) to prevent microbial adhesion and facilitate thorough cleaning.
- Crevice-Free Welding: Orbital welding ensures smooth, crevice-free joints that eliminate potential harborage sites for bacteria and biofilm.
- Comprehensive Monitoring: Advanced instrumentation for real-time monitoring of critical parameters like conductivity, TOC (Total Organic Carbon), temperature, and flow, ensuring continuous compliance.
- Easy Disinfection & Sterilization: Designed to be fully compatible with chemical or hot water disinfection/sterilization-in-place (SIP) procedures, crucial for maintaining aseptic conditions.
- Documentation & Traceability: Provided with extensive documentation for validation purposes, including material certificates, weld logs, and testing reports, supporting regulatory compliance.
This meticulous attention to design, material, and process ensures that the medical grade RO system is not only effective but also fully auditable and compliant with the stringent requirements of medical, pharmaceutical, and laboratory environments.
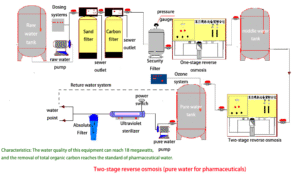
Reliability for Patient Safety and Critical Processes
The STARK Medical Grade RO System delivers unwavering reliability, a non-negotiable trait when patient safety or critical research is at stake. Its automated controls, robust components, and comprehensive design minimize the risk of contamination and system failure, providing continuous access to the highest purity water. This ensures optimal performance for dialysis machines, central sterile supply departments, laboratory analyses, and pharmaceutical manufacturing processes, contributing directly to positive patient outcomes and accurate scientific results.
Produkt-Parameter
Stark Water’s STARK Medical Grade RO System is engineered for the highest standards of water purity and reliability in critical medical applications. Here are its key technical specifications:
| Parameter | Spezifikation / Anpassungsoptionen |
|---|---|
| Hauptschlüsselwort Fokus | medical grade RO system design and capabilities. |
| Water Quality Output |
|
| System Configuration | Single or Double Pass Reverse Osmosis (RO) with integrated pre-treatment. Optional post-RO polishing (EDI, UV, Ultrafilters, Deionization). |
| Capacity | Customizable, ranging from 50 LPH to 5000+ LPH (or GPM equivalent), based on application demand (e.g., dialysis clinic, pharmaceutical plant). |
| RO Membrane Type | High-rejection Thin-Film Composite (TFC) RO Membranes, often low-fouling/sanitary-grade. |
| Construction Material (Wetted Parts) | High-grade Stainless Steel (SUS316L, mirror-polished internal surfaces Ra < 0.6 μm or < 0.4 μm). PVDF or PTFE for specific components. |
| Piping | Sanitary Stainless Steel (SUS316L, orbital welded where possible) or PVDF. |
| Disinfection Method | Compatible with Hot Water Sanitization (80-85°C) or Chemical Disinfection (SIP/CIP ready). |
| Control System | Fully Automatic PLC Control with HMI. Comprehensive monitoring of conductivity/resistivity at multiple points, flow, pressure, temperature, TOC, UV intensity. Alarms, data logging, and remote monitoring. |
| Pre-treatment System |
|
| Stromversorgung | 3 Phase, 380V/415V/460V, 50Hz/60Hz (Customizable). |
| Certifications & Standards | Designed and manufactured to meet industry standards like USP (United States Pharmacopeia), EP (European Pharmacopoeia), JP (Japanese Pharmacopoeia) for PW/WFI, AAMI (Association for the Advancement of Medical Instrumentation) for dialysis water, ISO, CE, FDA/GMP compliance. |
| Dimensions | Customized skid-mounted or modular design to fit cleanroom or facility requirements. |
Anwendbare Industrie
Die STARK Medical Grade RO System is widely adopted across various sectors where clean, microbiologically safe, and low-conductivity water is critical. Our Two-stage RO platform is flexible enough to support applications ranging from small clinic equipment to centralized hospital sterilization.
- Dialysis Centers: Provides high-purity feed water for hemodialysis machines. Two-stage RO ensures the removal of endotoxins, bacteria, and heavy metals to protect patient safety.
- Hospital CSSD (Central Sterile Supply Department): RO water is used for cleaning, rinsing, and steam generation in autoclaves, minimizing the risk of mineral deposits and microbial residue on surgical instruments.
- Clinical Laboratories: Supports reagent preparation, sample dilution, and device rinsing. Low TDS levels improve testing accuracy and extend analyzer lifespan.
- Pharmaceutical Production: Acts as pre-treatment before ultra-pure water systems or is used directly for non-injection cleaning and formulation processes.
- Research Institutes & IVF Centers: Ensures contamination-free water for cell culture, DNA extraction, and sensitive biological reactions.
- Mobile Medical Units / Field Clinics: Offers compact water purification solutions with generator/solar power options, suitable for remote or emergency healthcare deployment.
In each of these settings, STARK’s systems are customized to meet space limitations, operational load, and water safety regulations — offering both performance and peace of mind.
Vorteil Eins
In critical medical environments, water quality isn't optional—it’s essential. STARK’s dual-stage reverse osmosis architecture offers a robust solution to meet the highest purity standards for clinical and pharmaceutical use.
The first-stage RO system removes most dissolved solids, microorganisms, and organic substances. The second-stage RO further polishes the water by eliminating residual ions, pyrogens, and any potential microbial breakthrough from the first pass. This ensures stable production of ultrapure water with:
- Conductivity as low as 1–2 μS/cm
- Endotoxin reduction to below 0.25 EU/mL
- Total Dissolved Solids (TDS) removal rate > 98%
- TOC reduction < 50 ppb
With this Two-stage process, our systems offer consistent water quality for critical departments such as dialysis, CSSD, laboratories, and compounding pharmacies. The separation efficiency and safety margin help ensure full compliance with international medical water requirements.
Vorteil ZWEI
Verwandte Produkte
Ähnliche Produkte
FAQ
F1: Wie lange hält die Umkehrosmoseanlage?
Die Lebensdauer einer Umkehrosmoseanlage hängt von der Wasserqualität und der Wartung ab. In der Regel hält die Membran 2-3 Jahre, während das System selbst bei richtiger Pflege über 10 Jahre halten kann.
F2: Kann das System an die unterschiedlichen Wasserbedingungen angepasst werden?
Ja, alle unsere Systeme sind vollständig anpassbar. Wir analysieren Ihren Wasserbericht und Ihren Anwendungsbedarf, bevor wir maßgeschneiderte Lösungen anbieten.
F3: Welche Normen erfüllen die STARK Geräte?
Unsere Produkte sind CE- und ISO 9001-konform und können auf Wunsch so gestaltet werden, dass sie spezifischen regionalen Vorschriften oder Industrienormen entsprechen.
F4: Wie lange ist die Lieferzeit für STARK RO Systeme?
Standardsysteme werden innerhalb von 7-15 Tagen ausgeliefert. Bei kundenspezifischen Aufträgen kann sich die Lieferzeit je nach Komplexität auf 20-25 Tage verlängern.
F5: Bieten Sie technische Unterstützung oder Installationshilfen an?
Ja. Wir bieten eine vollständige technische Dokumentation, eine Fernanleitung per Video und können auf Wunsch Techniker ins Ausland schicken.
F6: Was ist in Ihrem Angebot enthalten?
Unser Angebot umfasst die komplette Umkehrosmoseanlage, die Schalttafel, die Pumpen, die Vorbehandlungseinheiten und alle erforderlichen Armaturen. Installationswerkzeuge und Ersatzteile können auf Anfrage mitgeliefert werden.








