Century egg wastewater treatment deals with extremely caustic brine (often near pH 14), high salinity, suspended solids, proteins and oil/grease from washing and peeling. This guide explains two proven process trains, safe pH control, design ranges you can bench-test, and what to monitor from start-up to steady state.
Why Century Egg Wastewater Treatment Is Challenging
- Very high pH from NaOH/Ca(OH)2 marinade — must be neutralized under control (never by dilution).
- High salinity (brine) — osmotic stress inhibits anaerobic/aerobic biology if the strongest streams are not segregated.
- Organics — proteins/peptides and marinade residues drive COD/BOD.
- SS & FOG — shell fines and grease often require front-end clarification or DAF.

Century Egg Wastewater Treatment Process Options
Option 1 — pH Adjustment → Coag/Floc → Anaerobic → Aerobic → Clarification
- Equalization (EQ): buffer flows/loads; mixer + level control; conductivity probe recommended.
- Neutralization: acid dosing (HCl or food-grade CO2) with dual pH probes and interlocked pumps; target pH 6.8–7.5 before coagulation.
- Coagulation/Flocculation: PAC/FeCl3 + polymer; add DAF when FOG is high or quick solids capture is needed.
- Anaerobic (UASB/AnMBR): high-rate COD removal and biogas; keep feed salinity within acclimated limits.
- Aerobic polishing (A/O, SBR or MBR): close BOD/SS; nitrification if ammonia is present.
- Secondary Clarifier / Membrane: achieve solids limit; optional sand/carbon filters for final polishing.
Strengths: low OPEX (biogas credit), good for medium–large flows. Watch-outs: longer HRT, biology needs salinity control.
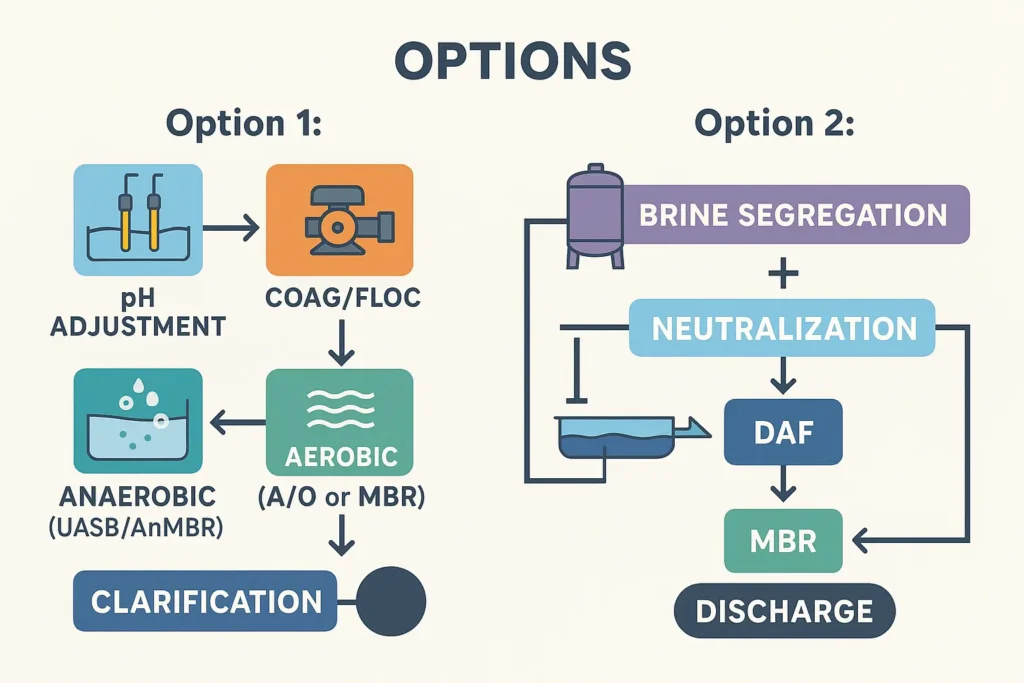
Option 2 — Brine Segregation Line + Physicochemical Mainstream + Aerobic MBR
- Brine-only line: collect the strongest marinade separately; do dedicated neutralization in a small, high-torque tank; controlled blending or partial desalination if TDS remains excessive.
- Mainstream line: wash water + light brine → neutralization → coag/floc → DAF.
- MBR: compact aerobic polishing with consistent low SS; no separate clarifier required.
Strengths: small footprint, faster start-up, robust to shift changes. Trade-off: higher CAPEX (membranes) and moderate OPEX.
| Artikel | Option 1 | Option 2 |
|---|---|---|
| Fußabdruck | Medium–large | Small |
| Start-up Speed | Slower (seed/acclimation) | Fast |
| Salinity Tolerance | Needs careful control | Good (split-stream) |
| OPEX | Low (biogas credit) | Mittel |
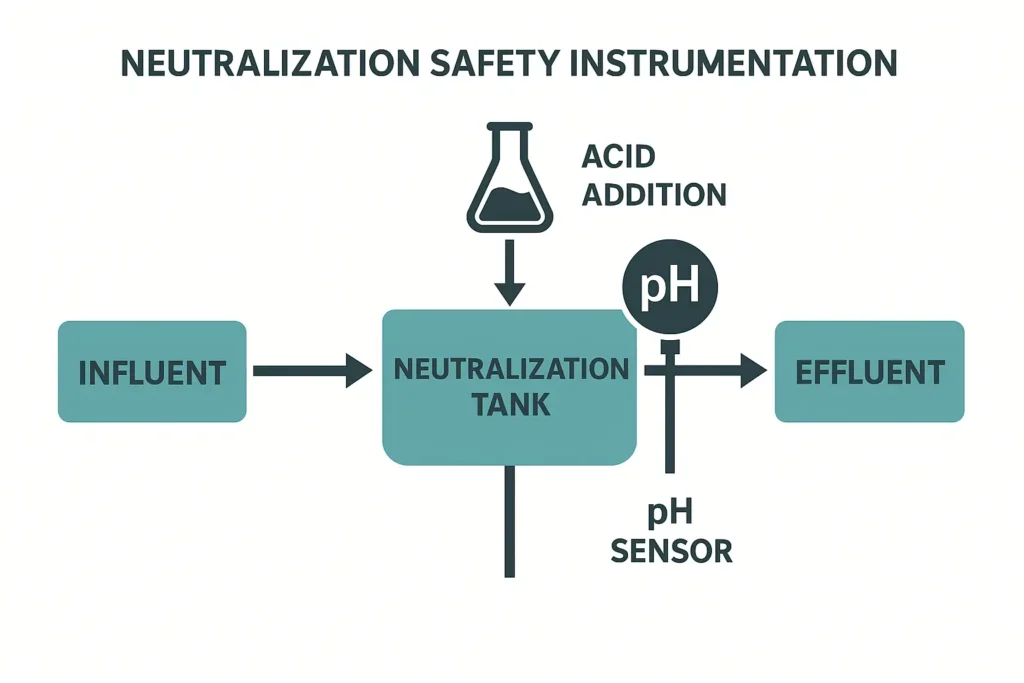
Safe Neutralization of pH-14 Brine
- Instrumentierung: dual pH probes (duty/standby), static or flash mixer, interlocked dosing pumps; high/low pH trips overflow back to EQ.
- Acid choice: HCl is compact and precise; CO2 is safer and reduces overshoot (forms bicarbonate). Use corrosion-resistant materials and ventilation.
- Control concept: coarse pH trim in EQ, fine trim in a dedicated neutralization tank just before coag/floc.
- Safety: reaction is exothermic; add acid to water; provide secondary containment and eyewash/shower as standard EHS practice.
How to Estimate Acid Demand (Titration → Full-Scale)
- Take a well-mixed 1 L sample.
- Titrate with HCl of known normality to pH 7.0; record the volume used: V_HCl (litres).
- For 1.0 N HCl: acid equivalents per litre = V_HCl × 1.0.
- Per m³ requirement = (acid equivalents per litre) × 1000. If using 10 N HCl, divide the result by 10 to get litres per m³.
Worked example (step-by-step): A 1 L sample needs 0.20 L of 1.0 N HCl to reach pH 7.0.
Acid equivalents = 0.20 × 1.0 = 0.20 mol/L.
Per m³ = 0.20 × 1000 = 200 mol H⁺ equivalent.
Using 10 N HCl: volume per m³ = 200 ÷ 10 = 20 L/m³.
Add 10–20% control margin; let the PID loop fine-trim.
Anmerkung: If you need to convert a supplier’s mass-percent HCl to normality, use the SDS values for wt% und density.
Approximation for HCl (monoprotic): N ≈ (density × wt% × 10) / 36.46 (density in g/mL; wt% as %). Example: 31% HCl at 1.15 g/mL → N ≈ (1.15 × 31 × 10) / 36.46 ≈ 9.8 N.
Typical Design Ranges (Bench-Test Before Final Design)
- EQ HRT: 6–12 h.
- Coag/Floc: flash mix \(G·t\) ≈ 300–1000; floc \(G·t\) ≈ 20,000–80,000; optimize by jar tests.
- DAF: surface loading \( \mathrm{SLR} = Q/A \) = 5–10 m³·m⁻²·h⁻¹; recycle 10–30%.
- UASB/AnMBR: organic loading \( \mathrm{OLR} = Q \times \mathrm{COD}_{in}/V \) = 1–6 kg COD·m⁻³·d⁻¹; acclimate for salinity.
- MBR: MLSS 6–12 g·L⁻¹; SRT 12–25 d; monitor TMP and set CIP triggers.
- Final pH: typically 6–9; COD/BOD/SS to meet your permit or local Standard A/B equivalents.
Operation & Monitoring
- Online: pH (dual), conductivity (salinity), DO in aerobic tanks, ORP in anaerobic, DAF interface level, MBR TMP.
- Routine lab: COD, BOD5, SS, oil/grease; record chemical usage and sludge volumes.
- Sludge: dewater (belt/plate); segregate brine-rich streams for licensed disposal.
FAQ
Do we need a separate tank for the strongest brine?
Yes. Segregation protects biology from salt shock and significantly shortens the overall HRT. A small neutralization tank dedicated to the brine line is best practice.
HCl vs CO₂ — which is better for neutralization?
HCl offers tight control with a small footprint. CO₂ is inherently safer and reduces overshoot; many plants use CO₂ for bulk trim and a small HCl dose for precise setpoint control.
How do we choose between Option 1 and Option 2?
If land is available and long-term OPEX matters most, choose Option 1 with anaerobic energy recovery. If space is tight, flows vary or rapid commissioning is needed, Option 2 with brine split + MBR is preferred.
Engineering Notice (Read Before You Design)
Values above are typical ranges from bench practice. Always verify with titration, jar tests and, where feasible, a small pilot before full-scale design. Do not substitute dilution for treatment. Install dual pH probes, dosing interlocks, ventilation, secondary containment and eyewash/shower per standard EHS practice.

