Last updated: November 4, 2025 · Reading time: 10–14 minutes · Audience: water plant operators, EPCs, utility engineers
Effectief RO pH control protects membranes, suppresses scaling, improves salt rejection, and stabilizes permeate quality. This guide explains carbonate chemistry, acid/caustic strategies, CO2 degassing, boron removal at high pH, instrumentation, and day-to-day O&M—so you can run reliably at the lowest lifecycle cost.
Why pH Is the Master Variable in RO
pH governs carbonate speciation (CO2 ⇌ H2CO3 ⇌ HCO3− ⇌ CO32−), directly affecting calcium carbonate/sulfate scaling, membrane charge, boron rejection, and corrosion. In practice, RO pH control pursues three outcomes: (1) extend membrane life, (2) meet product water targets, (3) minimize chemicals and energy.
Pretreatment pH Control: Break the CaCO3 Voorwaarde
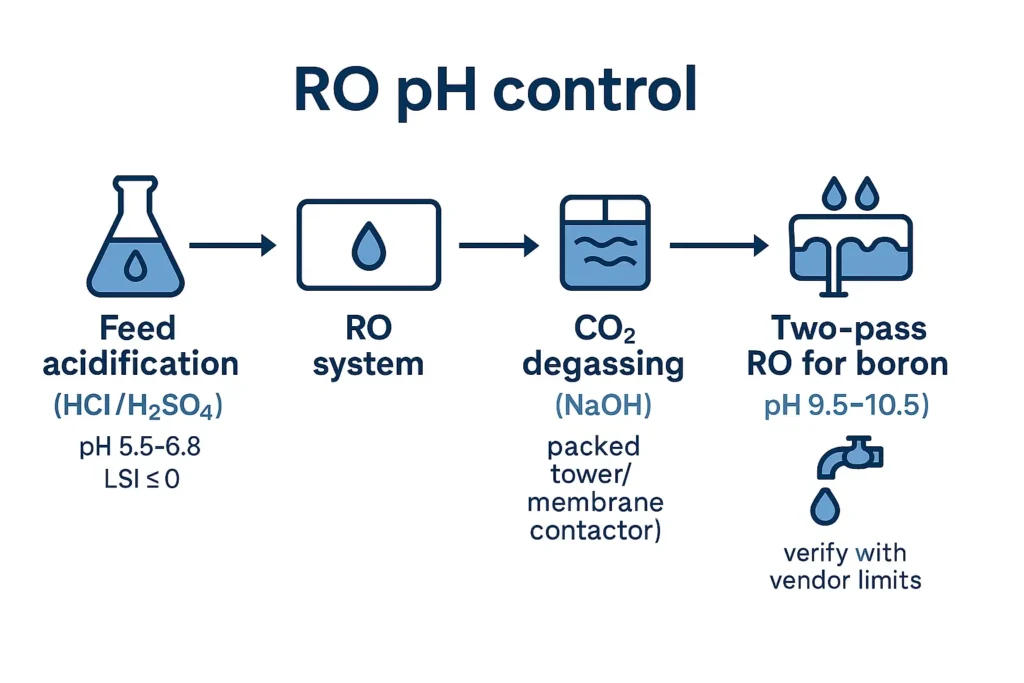
Above pH ≈ 8.3, carbonate shifts toward CO32− and CaCO3 is prone to precipitate. Acidifying the raw/RO-feed to roughly pH 5.5–6.8 (site-specific) pushes equilibrium to HCO3−/H2CO3, improving LSI and fouling safety margin.
- LSI targeting: move from positive to slightly negative/near-zero (e.g., −0.2 to +0.5 depending on vendor policy).
- Acid choices: HCl (no sulfate, more corrosive vapors) vs H2SO4 (cheaper, adds sulfate—watch CaSO4 index).
- Coagulation synergy: mildly acidic pH improves Fe/Al hydrolysis species and helps SDI reduction.
- Antiscalant vs acid: antiscalant controls multiple salts; acid primarily suppresses carbonates. Hybrid dosing is common at higher recoveries.
Tip: Verify LSI/CSI using your alkalinity, Ca2+, temperature, and TDS; log it against ΔP to see scaling trends. Useful references: U.S. EPA drinking water notes, WHO water quality.
Inside the Elements: What the Feed Gauge Won’t Tell You
Concentration polarization can raise the local concentration at the membrane surface several fold. The thin film’s chemistry often deviates from bulk feed—pH can locally increase, pushing surface LSI positive even when bulk LSI is near zero. Design levers to mitigate this include higher crossflow velocity, optimized spacers, staged recovery, and temperature control.
Permeate Chemistry: Why RO Permeate Is Often Acidic
CO2 slips through polyamide RO while alkalinity species are largely rejected; the resulting permeate often measures pH 4.8–5.5. Post-treatment options are (A) caustic neutralization and/or (B) CO2 stripping (degassing tower or membrane contactor) followed by trim caustic.
Route A — Caustic Neutralization
- Goal: raise product pH (e.g., to 7.0–8.0) and stabilize corrosion indices.
- Chemie: CO2 + NaOH → NaHCO3 (1:1 molar). Excess NaOH converts NaHCO3 → Na2CO3.
- Control: flow-paced NaOH dosing with PID trim from downstream pH; ensure static mixer and contact time.
Worked example (illustrative): neutralizing free CO2 with 30% NaOH
Given free CO2 = C mg/L, the required 100% NaOH is:
NaOH (g/m3) = C × (40/44).
For 30% NaOH solution, divide by 0.30; to get volume, divide by density (~1.33 kg/L).
Example: C = 20 mg/L CO₂
100% NaOH = 20 × (40/44) = 18.2 g/m³
30% NaOH mass = 18.2 / 0.30 ≈ 60.7 g/m³
30% NaOH volume ≈ 60.7 g / 1.33 g·mL⁻¹ ≈ 45.6 mL per m³
Opmerking: Real demand also depends on target pH, residual alkalinity, and CO2 stripping; confirm by titration.
Route B — CO2 Degassing (with Trim Caustic)
- Options: packed tower (counter-current air), forced draft decarbonation, or membrane contactor.
- Pros: lowers caustic consumption and reduces risk of overshooting pH.
- Cons: added footprint and fan/blower power; condensate management in humid climates.
Special Topic — RO pH Control for Boron Removal (Two-Pass)
Boron is weakly rejected as boric acid at neutral pH. Raising the second-pass feed to about pH 9.5–10.5 converts it to borate, which is better rejected. Guardrails: keep silica indices safe, and respect membrane pH limits (continuous vs CIP). Two-pass RO with interstage degassing is common in seawater and reuse projects.
Instrumentation & Control Architecture
- Probe map: raw → post-acid → RO feed → concentrate loop → permeate → post-alkali. Verify with routine lab checks and slope calibration.
- Derived control: online LSI/CSI from pH, alkalinity, EC, temperature; feed-forward acid setpoint with PID trim.
- Loop design: flow-paced dosing, duplex pumps, day tanks with high/low alarms, injection quills, static mixers, minimum contact time.
- Safety & materials: HCl/H2SO4/NaOH compatibility (PVC-C/PP/FRP/PTFE), splash shields, eyewash, double containment, venting.
- Vergrendelingen: dechlorination ORP, low-level chemical trip, high-pH/high-LSI inhibit, RO low-pH shutdown.
For general guidance on instrumentation and online monitoring, see European Environment Agency water topic.
Design Calculations & Sizing Aids
- Acid demand: size from titration curve/alkalinity (as CaCO3), target pH, and required LSI shift.
- Hydraulica: dosing point 10–20 pipe diameters upstream of membranes; provide static mixing energy (G·t) to avoid short-circuiting.
- Membrane limits: typical polyamide continuous ranges are narrower than CIP ranges—always confirm with the datasheet.
- Data logging: trend ΔP, recovery, LSI, pH setpoints, acid/caustic specific consumption (g/m3).
| Location | Primary Setpoint | Secondary Check | Opmerkingen |
|---|---|---|---|
| RO feed | pH 5.8–6.5 | LSI ≤ 0 | Balance vs antiscalant program |
| Concentrate loop | trend only | ΔP vs time | Indicates scaling/fouling onset |
| Permeate (post) | pH 7.0–8.0 | Alkalinity 10–40 mg/L | Adjust for end-use/corrosion |
| RO-2 (boron) | pH 9.5–10.5 | Silica index | Short duration high-pH with vendor approval |
O&M Playbook for RO pH Control
- Probe care: weekly slope check, monthly two-point calibration, keep wet caps and reference refills as specified.
- Chemical integrity: verify concentration (titration), install anti-siphon and back-pressure valves, label all lines and tanks.
- CIP windows: acid/alkali triggers by ΔP, normalized flux, and outlet EC; document soak time and rinse to neutral.
- Seasonal strategy: temperature shifts change equilibria and recovery—revisit LSI and dosing curves each season.
- KPIs: product pH, ΔP, recovery, LSI, specific chemical consumption, number of CIPs per 1000 h.
Troubleshooting by Symptom
Low permeate pH (4.8–5.5)
- Insufficient caustic or high CO2 slip → raise NaOH pace or add degassing.
- pH probe drift after CIP → recalibrate; check ground loops and cable shields.
Rising ΔP / suspected CaCO3 scale
- Feed pH/LSI above design → increase acid or reduce recovery.
- Antiscalant under-dosed → verify pump stroke and solution strength.
Unstable coagulation / metal carryover
- Over-acidification → re-tune metal coagulant dose and pH window.
Request a pH Control Plan
Share your last 6–8 weeks of feed data (pH, alkalinity, CO2, hardness, TDS, temperature) and product water targets. We’ll return a dosing & control scheme, instrument map, and budgetary CAPEX/OPEX.
Talk to an Engineer - Reverse Osmosis Solutions - See Case Studies
About the Author
Stark Water — Process engineers specialized in RO/NF, advanced oxidation, and reuse. We design, pilot, and operate plants with a focus on RO pH control, scaling prevention, and cost-optimized O&M.
Verder lezen: EPA water research - ISO water quality
FAQs — RO pH Control
1) What is the ideal RO feed pH for CaCO3 control?
Typically in the pH 5.5–6.8 range with LSI ≤ 0; fine-tune against recovery, temperature, and antiscalant program.
2) HCl or H2SO4—which should I choose?
HCl avoids sulfate scaling but is more corrosive to surroundings; H2SO4 is economical but raises CaSO4 risk. Model indices and check metallurgy before deciding.
3) Why is my permeate pH ~5 even when feed pH is 6–7?
CO2 permeates RO; alkalinity does not. Neutralize with caustic and/or strip CO2 via degassing.
4) How do I size a degassing tower vs. only caustic dosing?
Base it on free CO2 load, air-to-water ratio, packing performance, temperature, and desired outlet pH; then trim with NaOH.
5) How to raise pH for boron removal without triggering silica issues?
Operate RO-2 at pH 9.5–10.5 with vendor approval, manage silica index and consider intermediate degassing.
6) Where should I place pH probes and how often to calibrate?
Raw, post-acid, RO feed, concentrate, permeate, and post-alkali. Weekly slope check; monthly two-point calibration minimum.
7) What alarm bands protect membranes while keeping cost low?
High-pH/LSI inhibit at feed, low-pH shutdown to protect elements, high product pH alarm after NaOH, and chemical low-level trips.
8) How do I reconcile LSI with antiscalant programs?
Run both: keep LSI near zero and dose antiscalant targeted to your limiting salts; validate with normalized flux and ΔP trends.

