This guide covers aseptic stainless steel water tank applications with step-by-step operations for PW/HPW storage, including validated CIP/SIP, KPIs and troubleshooting.
Ready to specify or upgrade? Browse aseptic tank models, pair with our 1000 LPH RO system, and select RO membranes (4040/8040) such as ULP31-4040.
1) Executive Summary
- When to deploy: Use an aseptic tank when PW/HPW must hold buffer capacity with low bioburden risk and validated cleaning.
- Operational wins: validated CIP coverage, vent filter integrity, dead-leg control, trending with alarms, and documented changeovers.
- Quick spec: 304/316L, RA≤0.8 µm (≤0.4 µm for higher sanitary), crevice-free internals, spray device sized for coverage, 0.2 µm hydrophobic vent filter with P/V valve.
2) System Roles & Typical Flows
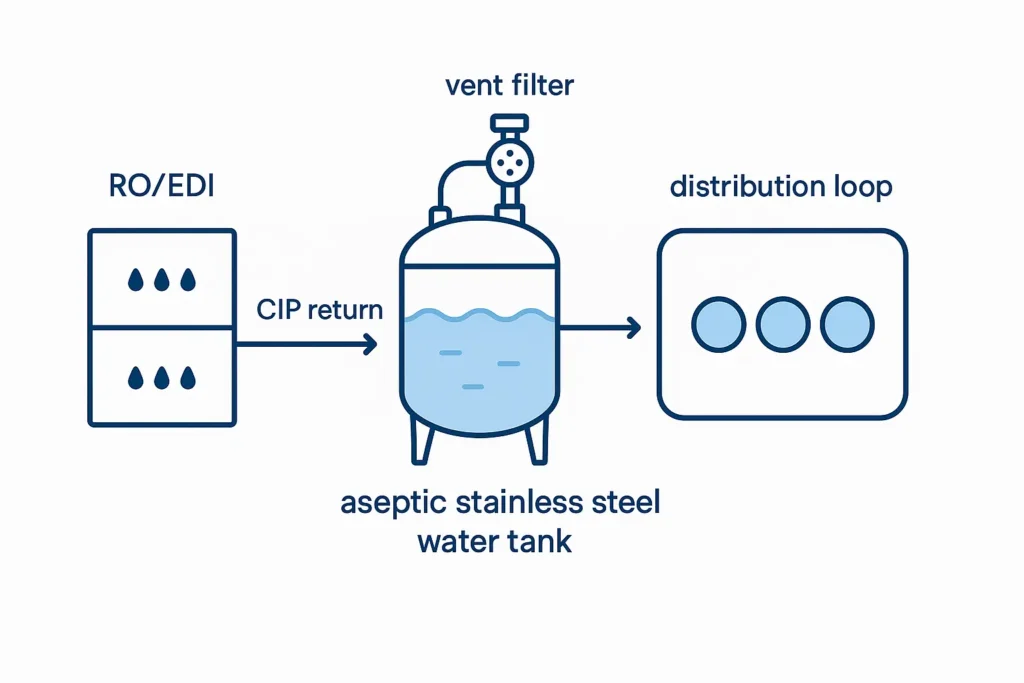
In most plants, the aseptic stainless steel water tank sits between RO/EDI and points of use, providing buffer, pressure stabilization and sometimes permeate blending. It also acts as the node for CIP return and SIP venting. Upstream packages like our 1000 LPH RO System and membrane selections from the membrane hub determine feed quality, recovery and CIP chemistries.
3) Applications by Industry
3.1 Pharma / Biotech (PW/HPW)
Tanks feed ambient or temperature-controlled loops. Validation focuses on surface finish, CIP coverage, vent filter integrity and trending. A well-run aseptic stainless steel water tank reduces bioburden excursions between RO/EDI and use points.
Spec checklist — 316L for chloride risk; RA≤0.4 µm for high sanitary; spray device sized for riboflavin coverage; 0.2 µm vent filter with integrity port; slope-to-drain; representative sampling valve.
CIP triggers — trend microbiology/conductivity; ATP/swab results; scheduled cycles by risk assessment.
Common pitfalls — wet vent filters, cold-spot misses during SIP, dead-legs on instrumentation tees.
3.2 Beverage & Dairy
Ingredient/blend water and CIP make-up rely on predictable tank hygiene. Seasonal water temperature swings and organic load challenge cleaning; jacket/insulation and recipes stabilize outcomes.
Spec checklist — 304 or 316L by product; RA≤0.8 µm (≤0.4 µm if required); rotary spray for heavy soils; insulated shell; odor control.
CIP triggers — product changeover, sensory/ATP alerts, scheduled sanitation.
Common pitfalls — protein/fat films, flavor carryover, under-heated cycles.
3.3 Cosmetics & Personal Care
Spec checklist — cross-contamination controls, compatible gaskets, validated rinse endpoints, batch traceability.
CIP triggers — formula class changes, surfactant films, visual residue.
Common pitfalls — fragrance adsorption, TOC spikes post-CIP, instrument drift.
3.4 Industrial Pure Water
Spec checklist — loop interlocks, conductivity alarms, bypass for maintenance, redundancy for critical tools.
CIP triggers — conductivity/TOC trend, scheduled shutdowns, biofilm indicators.
Common pitfalls — stagnation during outages, venting mistakes, vacuum collapse on cool-down.
4) Daily Operations: SOP & Checklists
4.1 Startup & Changeover
- Pre-use checks: vent filter ∆P, spray device status, valve positions, conductivity baseline.
- Flush/sanitize options per URS; document reasons for changeovers (code the cause).
- Record: date, batch/line, cleaning state, alarms since last run.
4.2 Shift / Daily / Weekly / Monthly Tasks
| Cadence | Task | Owner | Record |
|---|---|---|---|
| Shift | Visuals, conductivity & temp check; valve position | Ops | Log sheet |
| Daily | Spray device glance; vent filter ∆P; sampling (if required) | Ops/QA | LIMS / log |
| Weekly | Gasket inspection; alarm review; trend charts | Ops | CMMS ticket |
| Monthly | CIP performance audit; instrument verification | QA/Eng | Audit form |
4.3 Planned vs Condition-Based CIP/SIP
Run scheduled cycles but allow condition-based overrides—conductivity endpoints, ATP/swab results, and trending spikes. Document recipes and acceptance criteria; keep recipes version-controlled.
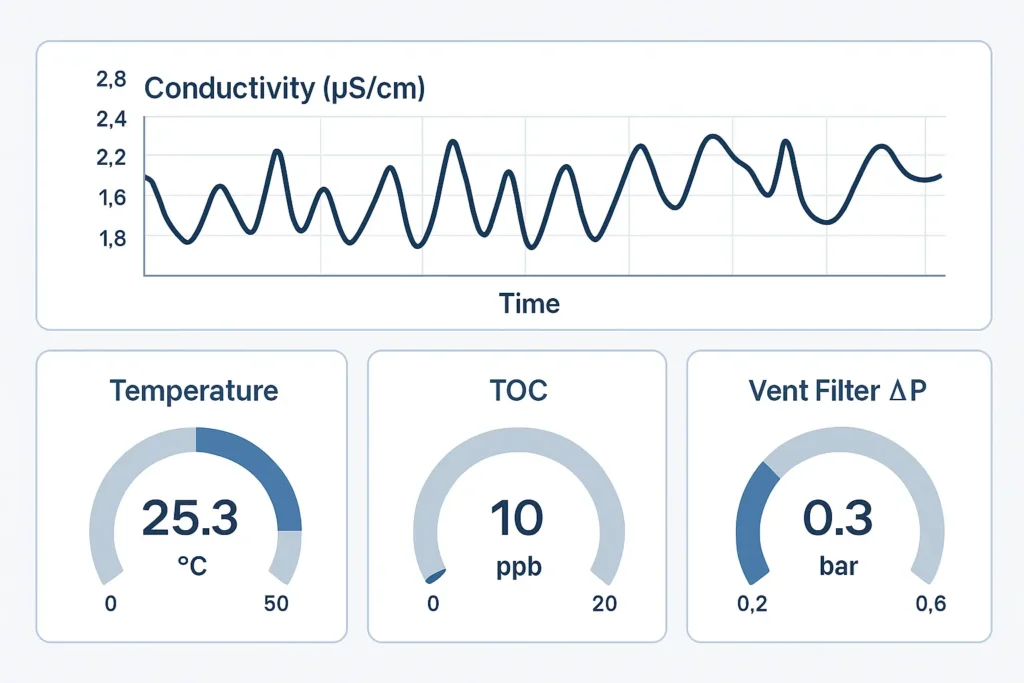
5) Monitoring & Alarms (KPIs that keep tanks clean)
- Conductivity (feed, tank, loop return) — rinse endpoints & stability.
- Temperature — cleaning energy and SIP hold.
- TOC (if specified) — organic residue watch.
- Micro trend — excursions vs action limits.
- Vent filter ∆P — wetting/blockage indicator.
- CIP profiles — flow/pressure/temperature curves.

6) CIP/SIP in Routine Operations (Actionable)
6.1 CIP baselines
- Sequence: pre-rinse → alkaline (40–70 °C) → rinse → acid (40–60 °C) → final rinse → optional passivation.
- Coverage: size pump & spray device to spec; verify with riboflavin at FAT and re-verify on change control.
- Endpoints: inline conductivity for rinses; add TOC where required.
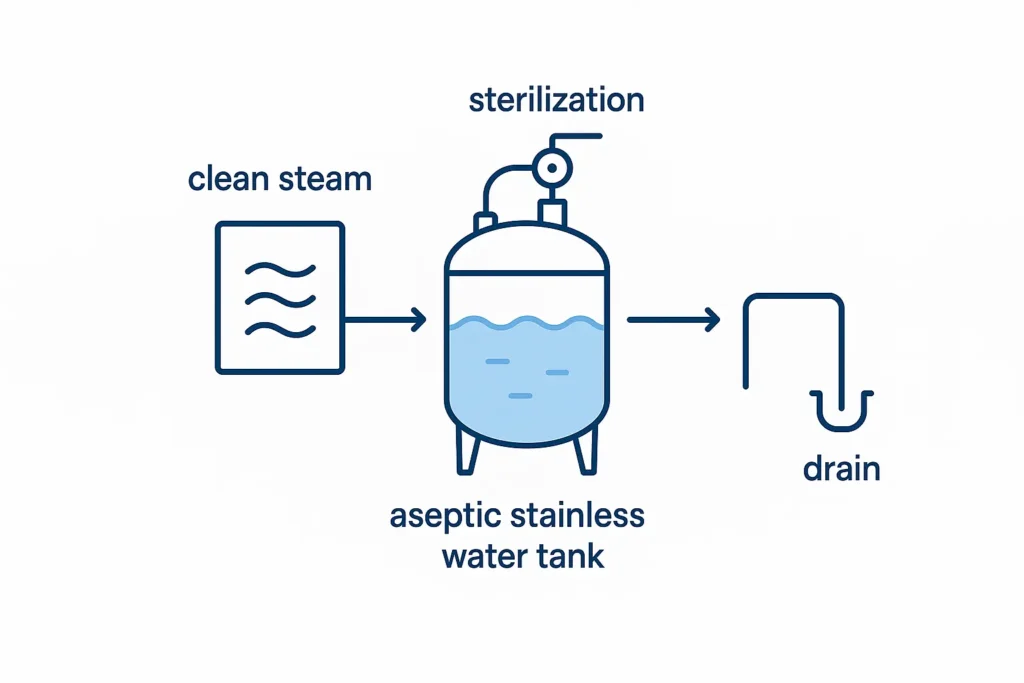
6.2 SIP essentials
- Typical regime ~121 °C hold 20–30 min measured at the cold spot.
- Venting via sterile filter; drain condensate; control cool-down to avoid vacuum collapse.
- Post-SIP: vent filter integrity test (bubble point) with records.
Related: 1000 LPH RO System • Water treatment packages • RO membranes 4040/8040
7) Troubleshooting & FMEA (fast trees)
7.1 Biofilm growth
Symptoms: micro/TOC drift; Causes: dead-legs, insufficient energy, long idle; Fix: hotter/longer recipes, dead-leg remediation, loop velocity checks.
7.2 Vent filter wetting / collapse
Symptoms: rising ∆P, vacuum events; Causes: condensate wetting, wrong P/V setting; Fix: heat tracing or preheat, proper cool-down curve, integrity test SOP.
7.3 Rouge/corrosion
Check chemistry compatibility, re-passivate or electropolish as needed; verify chloride and temperature limits.
7.4 Instrument drift
Schedule calibration; add dual-channel comparison for conductivity and temperature.
8) Cost & Efficiency (without risking hygiene)
- CIP solution recovery & re-use with quality limits.
- Balance concentration vs temperature for soil type.
- Combine batches/lines to reduce start-stop cycles.
- Track water/power/chemicals per CIP and benchmark.
| CIP Cycle | Water | Energia | Substancje chemiczne | Uwagi |
|---|---|---|---|---|
| Baseline | 1.0× | 1.0× | 1.0× | Reference |
| Recovered rinse | 0.75× | 0.95× | 1.0× | Reuse final rinse as pre-rinse |
| Optimized temp | 1.0× | 0.85× | 1.05× | Hotter/shorter where allowed |
9) Documentation & Compliance (Ops-first)
- Run logs, CIP/SIP batch records, vent filter integrity reports, change control, training matrix.
- QA pack: EN 10204 3.1 certs, weld map, WPS/PQR, surface roughness, hydrostatic test, FAT/SAT.
Useful industry references: 3-A Sanitary Standards, EHEDG hygienic design, ASME BPE overview.
10) 30–60–90 Day Adoption Plan
- 30d: SOPs live, KPI dashboard, CIP audit.
- 60d: FMEA fixes & root-cause actions; cost baseline.
- 90d: re-validation & optimization of CIP cadence; skills refresh.
11) Case Snapshots
- Pharma PW 10 m³: 316L, RA≤0.4 µm, validated CIP, vent filter integrity after SIP; excursions eliminated.
- Dairy blend 30 m³: rotary spray + jacket; seasonal stability achieved; weekly sanitation instead of daily.
- Electronics PW 5 m³: interlocks to tools; conductivity alarms; reduced downtime during maintenance.
12) Downloads & RFQ
Request a spec pack (GA + P&ID + Material List) tailored to your flow, finish and CIP/SIP needs.
13) FAQs
How often should an aseptic water tank be CIP’d in pharma?
Follow risk-based schedules—often weekly to monthly—plus condition-based triggers from micro/ATP and conductivity trends.
What triggers SIP vs chemical sanitization?
Select SIP when thermal lethality or validation requires it; use chemical sanitization for interim routines or temperature-sensitive plants.
How do I prevent vent filter wetting?
Preheat or heat-trace the vent path, drain condensate, control cool-down and confirm P/V valve settings; run post-SIP integrity tests.
What KPIs should I trend daily?
Conductivity (tank/loop), temperature, vent filter ∆P, micro trend; add TOC if specified.
Can I retrofit CIP/SIP ports and still pass validation?
Yes—issue change control, re-verify spray coverage (e.g., riboflavin), update recipes and acceptance criteria, and document results.

